Carbon disulfide:
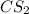
First, we need to know this:
1 mol of CS2 = 76.13 g (this value is obtained from the molar mass)
Also,
1 mol of CS2 = 76.13 g = 6.022 x 10^23 formula units ( molecules, atoms, etc.)
We have been asked for the number of molecules in 3.500 g of CS2.
Procedure:
75.13 g of CS2 ---------------- 6.022 x 10^23 molecules of CS2
3.500 g of CS2---------------- x
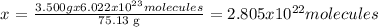
Answer: 2.805x10^22 molecules of CS2