Question
A sample of gas in a balloon has an initial temperature of 45 °C and a volume of 1.18x10^3 L. If the temperature changes to 82 °C, and there is no change of pressure or amount of gas, what is the new volume, V2, of the gas?
Express your answer with the appropriate units.
Procedure
Charles' Law gives the relationship between volume and temperature if the pressure and the amount of gas are held constant. This formula can be used because due to the high temperature the gas will have an ideal behavior.
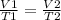
Rearranging the equation to determine V2 we have
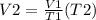
Solving the equation using the given values we have
V2= 1317.23 L or 1.317 x 10^3 L
Answer
V2= 1317.23 L