36 gloves represent 100%.
To find how many gloves represent 10%, we can use the next proportion:
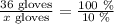
Solving for x:
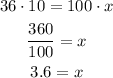
This result indicates that 3 gloves are less than 10%, so she should have ordered 4 gloves.
The percent error is computed as follows:
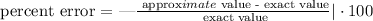
Substituting with data:
