Given data:
Mass of the block;
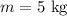
Coefficient of static friction;
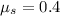
The static friction is given as,
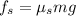
Here, g is the acceleration due to gravity.
Substituting all known values,
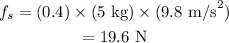
In order to just move to block the applied force must be greater or equal to the static friction force. Therefore, a 19.6 N of horizontal force must be applied to just start the block moving.