In order for steam to reach the body temperature, of about 37°C, first it will need to release the latent heat, which is the heat necessary to change from the steam state to the liquid state, and then the sensitive heat, which is actually responsible for the change in temperature.
The latent heat of water on the steam state is of 540 cal/g. Thus, we'll have:
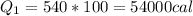
Now, we'll need the sensitive heat of water, which is 1 cal/(g°C):
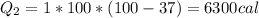
Thus, the total heat will be the sum of both, which will result in 60300cal