Okey, we are going to use the hlaf life formula, let's do it:
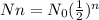
Where:
N=6
Nn = quantity remaining after n half-lifes
No = initial quantity=125
Replacing we obtain:
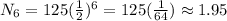
Finally we obtain that the radioactive isotope will have 1.95 grams after 6 half lives.