Answer: mass of HC2H3O2 = 300.25 grams.
Explanations and Calculations :
GIVEN
• 800g of H2O > 800/1000= ,0.8 Litres of water
,
• Concentration = 6.25 M
,
• Molar mass acetic acid = 60.05g/mol
(i) Calculate number of moles :
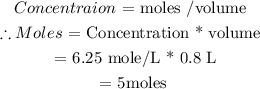
( ii) Calculate mass of CH3COOH or ( HC2H3O2)
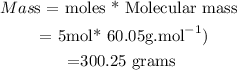
Therefore mass of acetic acid = 300.25 grams