Answer:
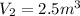
Step-by-step explanation:
Hello!
In this case, according to the Charles' law as a directly proportional relationship between volume and temperature:
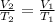
Thus, in order to compute the final volume, V2, we obtain the following expression:
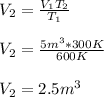
Best regards!