Chemistry => Kinetics => Reaction Rate
The reaction rate corresponds to the speed with which the reactants are consumed, or in other words the speed with which the products are formed.
From the graph, we can calculate the reaction rate by dividing the volume of products by the time. So, the reaction rate will be:
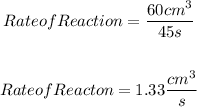
Answer: D. 1.33 cm3/s