Given, percentage of HCl in first solution, R1=20%.
Percentage of HCl in second solution, R2=55%.
The amount of second solution, A2=813.75 ml.
The percentage of HCl in mixture, R=20%.
Let A1 be the amount of first solution added to get mixture.
Hence,
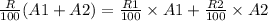
Now put the values in above equation.
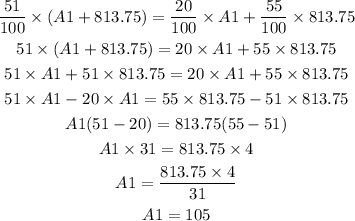
Therefore, 105 ml of 20% HCl is to be added to 813.75 ml of a 55% HCl to get 51% HCl solution mixture.