Step-by-step explanation:
We are given: mass of BaCl2 = 43.6g
: volume of BaCl2 = 100mL
: volume of BaCl2 = 230mL
We know: molar mass of BaCl2 =208.23g/mol
We determine the number of moles of BaCl2:
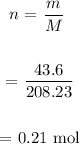
We then find the concentration of BaCl2:
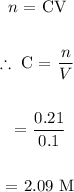
We then find the number of moles for a 230mL solution:
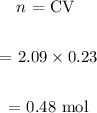
We then find the required mass:
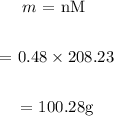
Answer:
100.28g must be added per 230mL of water.