Hello there. To solve this question, we'll have to remember some properties about half-life of carbon-14.
We can use the following formula to determine how many years has passed by finding how many half-life sessions the carbon-14 endured:
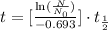
In which:
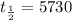
In this case, N is the amount of sample we have after the period t of time and N0 is what we started with.
Knowing that the fossil was found with 15% of its initial sample, this means that the ratio N/N0 = 15/100 = 3/20=0.15
Therefore we have:
Using a calculator, we find that ln(0.15) is approximately equal to -1.89712, thus
Multiply the numbers and round the answer to the nearest year: