Answer:
D. Kc = [Fe]² [H2O]³ / [Fe2O3] [H2]³.
Step-by-step explanation:
Let's suppose this hypothetical reaction:
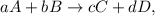
where the equilibrium constant is given by this expression:
![K_c=(\lbrack C]^c\lbrack D]^d)/(\lbrack A]^a\lbrack B]^b),](https://img.qammunity.org/2023/formulas/chemistry/college/x8skxwydvbqd2pgdkefyuq0gujmzi05irr.png)
The expressions in the brackets are the concentrations of the species of the reaction with their respective coefficient as exponents.
So based on this logic, the equilibrium constant expression for this case, will look like this:
![K_c=(\lbrack Fe]^2\lbrack H_2O]^3)/(\lbrack Fe_2O_3]\lbrack H_2]^3)](https://img.qammunity.org/2023/formulas/chemistry/college/2j9ggtcg54dqxvugwp41zc9m94c9ux43n3.png)
The answer would be D. Kc = [Fe]² [H2O]³ / [Fe2O3] [H2]³.