1) List known data.
Moles of sugar: 0.500
Mass of water: 0.750 kg
2) List unknown data.
Boiling point elevation:
3) Set the equation for boiling point elevation
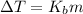
ΔT: change in temperature
Kb: Ebulloscopic constant (0.512 ºC/molal).
m: molality.
4) Molality of the solution
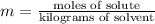
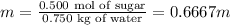
5) Plug in values in the equation
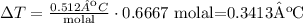
The change in the boiling point is 0.341ºC.