ANSWER
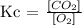
Step-by-step explanation
Given that
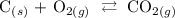
Follow the steps below to write the equilibrium expression for the reaction
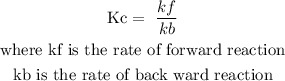
When writing equilibrium expression, we don't include substances that exist in solid state.
Hence, we have
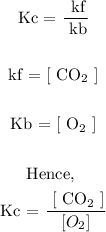
Therefore, the correct option is B