Step 1 - Remembering the law of mass conservation
As stated by the law of mass conservation, in a chemical process no atoms can be created or destroyed. Consequently, the mass of the system will not change: the sum of the masses of reactants will be exactly equal the sum of the masses of products.
Step 2 - Using the law of mass conservation to understand the problem
The law of mass conservation therefore guarantees that, if we are mixing 7.2 g of C with 19.2 g of O, we will form 7.2+19.2 g of whatever is the product. Therefore we will form 26.4 g of product, which will be either CO or CO2.
Step 3 - Using mass percentage to find the correct product
Since we already know what will the mass of the product be, we can calculate the percentage of C in the product:
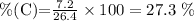
Now, let's calculate the percentage of C in both CO (28 g/mol) and CO2 (44 g/mol), remembering that the molar mass of C is 12 g/mol:
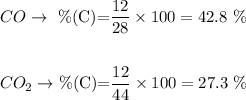
We can see that in CO2 the amount percent of C is exactly the same as we have calculated for the unknown product of this reaction. Therefore, the product is CO2.