1) Find the molar mass (MM) of glucose
C: 12.0107 g/mol
H: 1.00794 g/mol
O: 15.9994 g/mol
MM C6H12O6 = (6*12.0107) +( 12*1.00794) + (6*15.9994) = 180.15588 g/mol
2) Find the mass of carbon in glucose
12.0107*6 = 72.0642
3) Percent composition
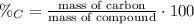
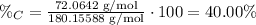
The percent composition of carbon in glucose is 40.00%.
.