Step-by-step explanation:
We are given: moles of Al(NO3)3 = 4 mol
: moles of NaCl = 9 mol
We know: molar mass of Al(NO3)3 = 212.996 g/mol
: molar mass of NaCl = 58.44 g/mol
: molar mass of NaNO3 = 84.9947 g/mol
The balanced chemical equation is given as:
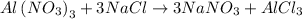
Number of moles of NaNO3 from Al(NO3)3:
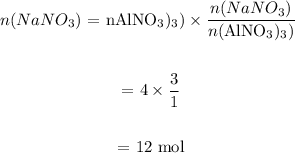
Number of moles of NaNO3 from NaCl:
Therefore, NaCl is a limiting reagent.
Answer:
The maximum amount of NaNO3 is 9 mol.
By balancing the chemical equation of the given reactants and products. And then use molar ratios and the number of moles to find the limiting reagent.