The reaction is balanced, so we can continue with the calculations.
We will first find the moles present in the KIO3 solution. For this we are going to use the molarity equation that tells us:
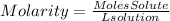
We solve the moles of solute from the equation:
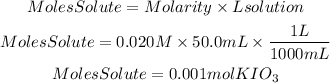
Now, they tell us that the rest of the reactant is in excess. therefore, the calculations will be made based on the moles of KIO3. By stoichiometry, we have that the I2 to KIO3 ratio is 3/1. So the moles of I2 formed will be:
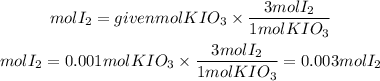
Now, the mass of I2 will be:
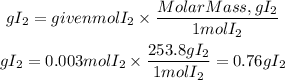
Answer: The mass of iodine formed is 0.76 g I2. Second option