Step-by-step explanation
Step 1
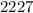
a)nearest ten
If the number you are rounding is followed by 5, 6, 7, 8, or 9, round the number up.
so,In this case, the digit to the right (7) is 5 or above. So, we add 1 to the tens place (2). The digit(s) at the right (7) becomes 0, thus we get 2230 as answer.
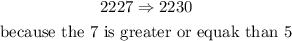
b)Nearest hundred
Remember that the hundreds place is three moves from the left of the decimal point (if it exists). To round to the nearest hundred (nearest 100), we use the tens place to determine whether the hundreds place rounds up or stays the same,so
in this case: 27
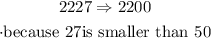
c)nearest thousand
Remember that the thousand place is four moves from the left of the decimal point (if it exists). To round to the nearest thousand (nearest 10000), we use the hundreds place to determine whether the thousands place rounds up or stays the same
so,in this case:227
Step 2
now, for :
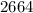
a) nearest ten
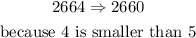
b)nearest hundred
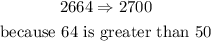
c)nearest thousand
I hope this helps you