We are told that the ratio of 1.4 to 32 is the same as the ratio of 2.8 to a value that we are going to all x. Then we can formulate the following expression:
ratio 1 = ratio 2
Where ratio 1 is the ratio of 1.4 to 32 and ratio 2 is the ratio of 2.8 to x. the ratio 1 can be expressed like this:
ratio 1 = 1.4/32
And the ratio 2 can be expressed like this:
ratio 2 = 2.8/x
Then, we get:
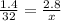
From this expression, we can solve for x to get:
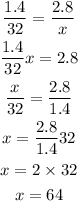
Then, the ratio of 1.4 to 32 is the same as the ratio of 2.8 to 64