ANSWER
The volume of CO2 used at STP is 77.325L
Step-by-step explanation
Given that;
The mass of C6H12O6 is 622g
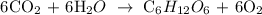
Follow the steps below to find the volume of CO2 reacted
Step 1; Find the number of moles of C6H12O6 using the below formula
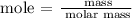
Recall, that the molar mass of C6H12O6 is 180.16 g/mol
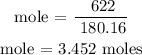
The moles of C6H12O6 is 3.452 moles
Step 2; Find the number of moles of CO2 using a stoichiometery ratio
In the above equation, 1 mol CO2 reacted to give 1 mol C6H12O6
Since 1 mol of CO2 is equivalent to 1 mole of C6H12O6
Therefore, the number of moles of CO2 is 3.452 moles
Step 3; Find the volume of CO2 at STP
Recall, 1 mole of a gas is equivalent to 22.4 L/mol
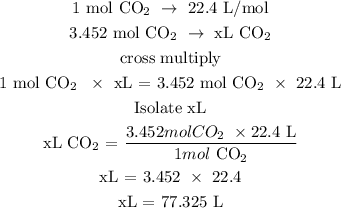
Therefore, the volume of CO2 used at STP is 77.325L