Answer:
2.06L
Explanations:
According to charle's law, the volume of a given mass of gas is directly proportional to its temperature provided that the temperature is constant. Mathematically;
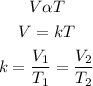
Given the following parameters
Initial volume V1 = 2.0L
Initial temperature T1 = 2.0°C = 2 + 273 = 275K
Final temperature T2 = 10 + 273 = 283K
Required
New/Final volume of the gas V2
Substitute
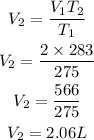
Hence the new volume of the gas is 2.06L