Answer: We can translate the given chemical equation as:
"potassium permanganate combined with zinc chloride produces zinc permanganate and potassium chloride". The best option to answer the question is the first one.
Step-by-step explanation:
The question requires us to choose, among the options given, the correct "translation" for the following chemical equation:
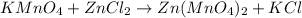
To solve this problem, we can separate the compounds into their cations and anions, and then analyze the metal (cation) name and anion name. Keep in mind that the name of the compound is formed by cation/metal name + anion name.
1) Let's start with KMnO4, which is formed by the cation K+ (potassium) and anion (MnO4)- (permanganate). The name of KMnO4 is potassium permanganate.
2) Next, let's analyze ZnCl2, which is formed by the cation Zn2+ (zinc) and anion Cl- (chloride). The name of ZnCl2 is zinc chloride.
3) Now, let's check Zn(MnO4)2, which is formed by Zn2+ (zinc) and (MnO4)- (permanganate). The name of Zn(MnO4)2 is zinc permanganate.
4) At last, let's analyze KCl, which is formed by K+ (potassium) and Cl- (chloride). The name of KCl is potassium chloride.
Therefore, we can translate the given chemical equation as:
"potassium permanganate combined with zinc chloride produces zinc permanganate and potassium chloride". The best option to answer the question is the first one.