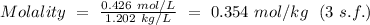Answer:
0.354 molal
Step-by-step explanation:
The molarity (M) or the concentration of a solution is defined as the number of moles of a compound in the solution per liter of solution (mol/L), whereas molality, is defined as the number of moles of a compound in the solution per kg of the compound (mol/kg).
Given that the density of the solution is 1.202 g/mL, which is equivalent to 1.202 kg/L. Since the prefix mili- denotes a factor of one thousandth (
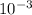 ) and kilo- denotes a factor of one thousand (
) and kilo- denotes a factor of one thousand (
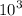 ),
),
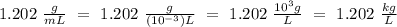 .
.
To calculate the corresponding molality of the solution, the formula
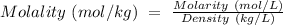 is used.
is used.
Therefore,