The first step to answer this question is to convert the given mass (68g LiNO3) to moles using the molar mass of lithium nitrate:
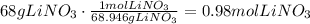
According to the given equation, 4 moles of LiNO3 produce 1 mole of Pb(NO3)4, the next step is to use this ratio to determine how many moles of Pb(NO3)4 are produced:
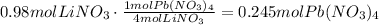
It means that 0.245 moles of lead (IV) nitrate are produced.