Answer:
The weight of the residue = 45.1 g
Step-by-step explanation:
The equation of reaction is:
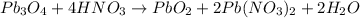
The residue will contain a mixture of PbO₂ and Pb(NO₃)₂
Molar mass of Pb₃O₄ = (207 x 3) +(16 x 4) = 685 g/mol
Number of moles of Pb₃O₄ = Mass/Molar mass
Number of moles of Pb₃O₄ = 34.3/685
Number of moles of Pb₃O₄ = 0.05 moles
Number of moles of HNO₃ = 4 x 0.05 = 0.2 moles
Molar mass of HNO₃ = 1 + 14 + (16 x 3) = 63 g/mol
Mass of HNO₃ = 0.2 x 63 = 12.6 g
Number of moles of H₂O = 2 x 0.05 = 0.1 moles
Molar mass of H₂O = (1 x 2) + 16 = 18 g/mol
Mass of H₂O = 0.1 x 18 = 1.8 g
Mass of the residue = Mass of Pb₃O₄ + Mass of HNO₃ - Mass of H₂O
Mass of the residue = 34.3 g + 12.6 g - 1.8 g
Mass of the residue = 45.1 g
Therefore, the weight of the residue = 45.1 g