Given:
The mass of water is: m = 200 g
To find:
The heat necessary to vaporize 200 g of water at 100°C to form steam at 100°C.
Step-by-step explanation:
The heat Q, mass m, and the latent heat L are related as:
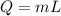
The latent heat of the water to convert it from 100°C to form steam at 100°C is 2260 J/g. Thus,
L = 2260 J/g
Substituting the values in the above equation, we get:
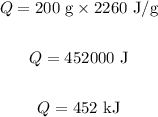
Final answer:
Thus, 452 kJ of heat will be required to vaporize 200 g of water.