Given:
The mass of water is m = 200 g
The initial temperature of the water is
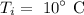
The final temperature of the water is
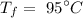
Required: The amount of heat needed to increase the temperature.
Step-by-step explanation:
The amount of heat can be calculated by the formula
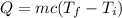
Here, c is the specific heat of the water whose value is
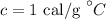
On substituting the values, the amount of heat can be calculated as
