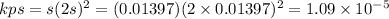Answer
Kps=1.09 x 10⁻⁵
Procedure
First, we will need to consider the solution equilibrium. In this case, we have the following equation that depicts the equilibrium.
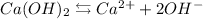
The molar solubility will be given by the following equation If only 0.238 g of Ca(OH)₂ is dissolved in enough water to give 0.230 L
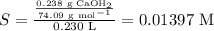
Therefore the Kps value will be determined as :