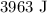Given:
the internal energy of the gas is
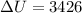
The work done on the gas is
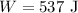
Required: heat needed to increase the internal energy
Step-by-step explanation:
from the first law of the thermodynamics
we can write,
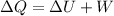
Plugging all the values in the above relation, we get
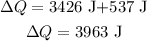
Thus, the heat needed