Step 1
Oxygen gas is assumed to be an ideal gas, so it is applied:
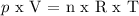
p = pressure
V = volume
n = number of moles
R = gas constant
T = absolute temperature
------------------
Step 2
Information provided:
V = unknown
p = 1.00 atm
n = 2.00 moles
T = 273 K
----
Information needed:
R = 0.082 atm L/mol K
------------------
Step 3
From step 1, V is found as follows:
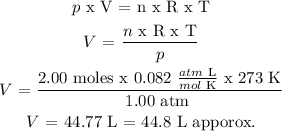
Answer: 44.8 L