Answer:
72.66g of Fe2O3 are produced.
Step-by-step explanation:
1st) From the balanced equation we know that 2 moles of Fe2O3 are produced from 4 moles of iron (Fe). With a mathematical rule of three we can calculate the moles of Fe2O3 that will be produced from 0.89 moles of iron:
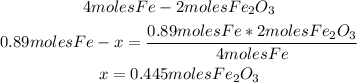
Now we know that 0.455 moles of Fe2O3 are produced.
2nd) Now we have to convert 0.455 moles of Fe2O3 into grams, by using the molar mass of Fe2O3 (159.7g/mol):
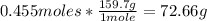
So, 72.66g of Fe2O3 are produced.