The reaction is written as:
2 NOCl (g) <=> 2 NO (g) + Cl2 (g)
Initial concentration of NOCl = 1 mol/ 1 L = 1 M
9.0 % is decomposed => 0.09 x 1 M = 0.09 M
It remains (equilibirum) = 1 M - 0.09 M= 0.91 M
---------------------------
Inicial concentration of NO and Cl2 is equal to 0
In equilibrium:
For NO) +2x = 0.09 M (is formed) => x = 0.045 M
For Cl2) +x = 0.045 M
---------------------------
Equilibrium concentrations:
For NOCl = 0.91 M
For NO = 0.09 M
For Cl2 = 0.045 M
---------------------------
K is calculated as (Kc):
Kc =
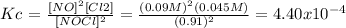
Remember: for the next reaction: a A + b B <=> c C + d D:
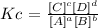
Answer: K = Kc = 4.40x10^-4