Answer:
![2PO_4^(3-)+3Zn^(2+)\operatorname{\rightarrow}Zn_3(PO_4)_2.]()
Step-by-step explanation:
Let's remember the concept of net ionic equation: the net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction.
The first step is to write the equation with dissociated ions. This means that we can 'separate' the compounds by their ions, like this:
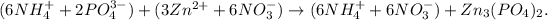
The next step is to 'cancel' those terms that are 'repeated' on both sides, for example, 6 moles of NH4 (+) and 6 moles of NO3 (-) are repeated, so the net ionic equation would be:
![2PO_4^(3-)+3Zn^(2+)\operatorname{\rightarrow}Zn_3(PO_4)_2]()