The density of any substance corresponds to the volume occupied by a given mass. Density varies with temperature and pressure. In this case, we will assume that the temperature and pressure remain constant, therefore the density will also remain constant. To calculate the density we can apply the following equation
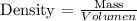
We have the following data:
Mass = 1.81 kg = 1810 g
Volume = 1435 cm^3 = 1435 mL
We replace the data into the equation,
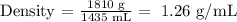
The density of the liquid is 1.26 g/mL
If we compare the density obtained with the measured compounds, we can verify that the glycerol has the same density.
Despite this, we cannot be sure that it corresponds to this substance, since as the container is not marked, the substance may contain a mixture of the substances contained in the rest of the bottles.
When mixing different substances with different densities, the final density will be an average, so it could also happen that by chance the density is equal to that of glycerol. Further laboratory analysis should be done to identify the unknown substance