ANSWER
Sodium = 1+
Step-by-step explanation
Sodium (Na) is a group 1 element with an atomic number of 11
Below is the electronic configuration of sodium
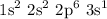
The above configuration shows that sodium has an electron at the outter most shell of the atom which is the valence electron.
For sodium atom to attain it octet state, it needs to release the single electron at it outter most shell
Hence, the ionic charge of sodium (Na) is 1+