To answer this question we have to usethe ideal gas law:

Where P is the pressure, V is the volume, n is the number of moles, R is the constant of ideal gases (0.082atmL/molK) and T is the temperature (in Kelvin degrees).
The first step is to convert the given mass of N2 to moles using its molecular mass:
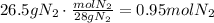
And convert the pressure from mmHg to atm (1atm=760mmHg):
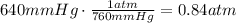
Finally, solve the initial equation for T and replace for the given values:
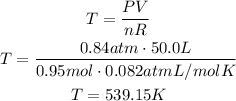
The temperature of the gas is 539.15K.
Convert this temperature to Celsius by substracting 273.15 to the temperature in Kelvins:
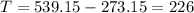
The temperature of the gas is 226°C.