Answer:
We have the following chemical equation:
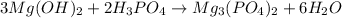
The first thing we do is chech if the equation is balanced. For this we count how many atoms of each element we have on each side of the equation:
Mg: 3
O: 14
H: 12
P: 2
So the equation is balanced.
Now, we transfor the mass of magnesium hydroxide and water into moles, by using their molar mass (M):
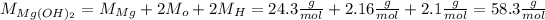
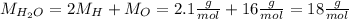
Now we calculate the number of moles of water (n) that we have to produce:
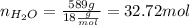
Now, looking at the chemical equation we can see that every 3 moles of magnesium hydroxide that react produce 6 moles of water. So in order to produce 32.72 moles of water we need:
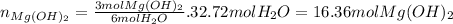
Finally we transform the moles into grams:
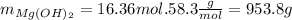
So the answer is 953.8g of magnesium hydroxide.