The expression for the number of seats S in a row r is given by:
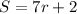
For r=1 we have:
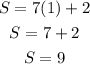
For 1 row we have 9 seats.
So far, this works, because the image has 9 seats in the first row.
For r=2 we have:
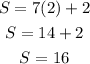
16 seat for the second row. This does not work, because in the image we have 11 seats in the second row.
For r=3 we have:
It also does not work. Because in the image we have 13 seats in the 3rd row, not 23.
So the equation of Jonathan does not work to model the number of seats.