ANSWER
The final volume is 90.45mL
Explanation
Given information
The initial volume of the gas = 61mL
The initial temperature of the gas = 29 degrees Celcius
The final temperature of the gas = 43 degrees Celcius
From the given data, you will see that the pressure of the gas is constant.
To find the final volume of the gas, we will need to apply Charle's law
Charle's law state that "the volume of a given mass of a gas is directly proportional to its temperature, provided that the temperature of the gas remains constant"
This law can be translated mathematically below

where
V = volume
T = temperature
From the above mathematical expression, we can now find our final volume
Let the final volume be V2
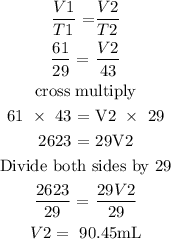
Therefore, the final volume is 90.45mL