ANSWER
The number of atoms of zinc in 102.26 grams is 9.42 x 10^23 atoms
Step-by-step explanation
Given that;
The mass of zinc is 102.26 grams
Follow the steps below to find the number of atoms
Step 1; Find the mole of the zinc atom using the formula below
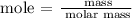
Recall, that the unit mass of zinc is 65.38u
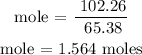
Step 2; Find the number of atoms of zinc using the below formula
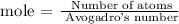
Recall, that the Avogadro's constant is 6.022 x 10^23
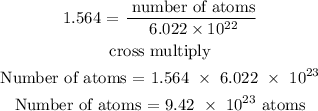
Therefore, the number of atoms of zinc in 102.26 grams is 9.42 x 10^23 atoms