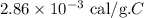Given:
The mass of the sample is,
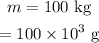
The temperature changes from
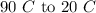
The heat rejected is,
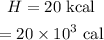
To find:
The specific heat capacity of the metal
Step-by-step explanation:
Let the specific heat capacity is 'c.'
The amount of heat rejected by the metal is,
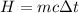
Here, the temperature change is
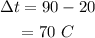
Substituting the values we get,
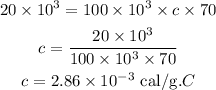
Hence, the specific heat capacity is,