Given:
A particular isotope has a half-life of 81 days.
Required:
If the starting amount is 1 kilogram of this isotope, find the remaining amount after 160 days, after 320 days.
Explanation:
The amount for exponential decay using the half line is given by the formula:
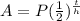
Where A =accumulated amount
P = initial amount
t = elapsed time
h = half time
We have P = 1
h = 81
Find A when t = 160
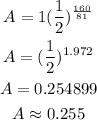
Find A when t = 320
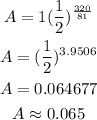
Final Answer:
The remaining amount after 160 days is approximately 0.255 kg
and after 320 days is approximately 0.065 kg.