ANSWER
320 mL
Step-by-step explanation
Let x be the amount of the 65% dextrose solution. The original solution contained 480 mL of a 20% solution and the amount x we take from that solution, is the same amount we have to add of the 65% solution to obtain the 50% concentration required.
The amount of the 20% solution left in the bag will be (480 - x). The 20% of this amount plus the 65% of x should be equal to the 50% dextrose in the final 480 mL solution. We have to solve the equation,
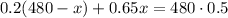
Apply the distributive property to eliminate the parenthesis and solve the product on the right side of the equation,
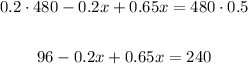
Add like terms,
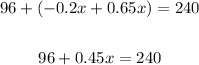
Subtract 96 from both sides,
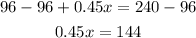
And divide both sides by 0.45,
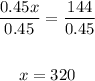
Hence, to get a 50% dextrose solution we have to replace 320 mL of the original solution with a 65% dextrose solution.