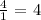Answer:
Step-by-step explanation:
Here, we want to write an acidic ratio
We start by balancing the ionic equation as follows:
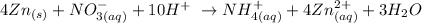
From what we have here, we have to check for the balance in terms of the number of atoms and the ions
From the balanced equation, we can see that 4 atoms of zinc reacts with a single ionic entity of the nitrate V
We have the ratio as: