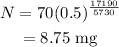Given data
*The carbon-14 has a half-life is t = 5730 years
*The amount of quantity contained is n = 70 mg
*The given time is T = 17190 years
The expression for the radioactivity decay equation is given as
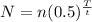
Substitute the values in the above expression as