To solve this question we have to use the following formula:
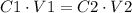
Where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration and V2 is the final volume.
From this we know the initial concentration, the final concentration and the final volume, we have to find the initial volume (V1):
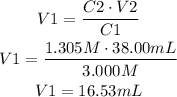
16.53mL are needed to make up 38.00mL of 1.305M.