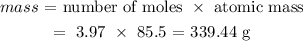Answer:
Step-by-step explanation:
Here, we want to get the mass in the given number of atoms
Firstly, we need to get the number of moles in the given number of atoms
Mathematically:
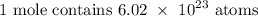
The number of moles in the given number of atoms would be:
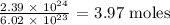
To get the mass, we need to multiply this number of mole by the atomic mass of Rubidium
The atomic mass of rubidium is 85.5 amu
Thus, we have the mass as: