We can find the volume of the solution using the formula of molarity:
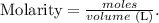
We have already the molarity (0.0550 M (mol/L) ) and the number of moles (0.163 moles). Clearing the formula for liters and replacing the data that we have, we're going to obtain:

The answer is that we have 2.96 liters in a 0.0550 M of LiBr solution with 0.163 moles.